Part 5: Getting people vaccinated
5.1
In this Part, we describe what has happened so far to prepare for the nationwide roll-out of the immunisation programme and what we understand was left to do at the time our audit was completed. We outline:
- the decisions made to establish the sequence in which people will be vaccinated;
- where vaccinations will take place;
- how vaccines will be delivered;
- who will administer them; and
- the work to prepare the technology systems required to support the roll-out.
5.2
For the immunisation programme to be ready for the roll-out to groups three (people at risk of severe outcomes from catching Covid-19) and four (the remainder of the population), we would expect the Ministry to have a clear understanding of the sequence in which people will be vaccinated and clarity about where vaccinations will occur. We would also expect the Ministry to have worked out the logistics to make sure that the correct amount of vaccine doses is available at the right place at the right time.
5.3
We would expect that the workforce that will be administering the vaccinations would have been identified and that plans are in place to ensure that they are adequately trained by the time the roll-out to groups three and four starts. We would expect key systems to be in place, or will shortly be in place, to ensure that they can be tested and adjustments made in time to avoid delays to the roll-out plan.
Summary of findings
5.4
As noted in paragraph 1.4, a sequencing framework has been developed that divides people into four groups. Vaccination of the first two groups is under way. It has been stated that group three will start being vaccinated in May 2021, and group four from July 2021. More detailed plans show that most people in groups three and four will receive their vaccines later than this, over the rest of 2021.
5.5
As of 15 April 2021, district health boards’ plans showed that they anticipate to have administered about 1.1 million doses by the end of June 2021.
5.6
Most people in group three will not be vaccinated before the end of June 2021. There will be an overlap of the vaccination of groups three and four. Planning documents show that most vaccines for group four will be administered between September and November 2021. In our view, although groups three and four may start as scheduled, there is a risk that these groups will not be fully vaccinated by the end of the year.
5.7
The Ministry has identified four types of locations for vaccinations to be administered. These are hospitals, temporary sites, community sites, and fixed vaccination centres. District health boards will choose which settings are the best options for each of their communities.
5.8
Equity considerations have been factored into the sequencing framework and the delivery models. District health boards are required to include in their delivery plans how they will ensure that Māori and Pasifika communities and people with disabilities will receive equitable access to the vaccine. Funding has also been set aside to increase access and uptake and to assist informed consent for Māori and Pasifika.
5.9
At the time we completed our audit, some of this funding had been distributed to Pasifika health care providers but decisions were still to be made about how this funding will be allocated to Māori providers. Continued delay means those health care providers will have very little time to prepare for the roll-out.
5.10
The workforce required to administer and support vaccinations is greater than has been needed for previous immunisation programmes. It also needs to be deployed in a way that does not result in major diversion of staff from other public health and immunisation programmes. The Ministry plans to use both the regulated and unregulated workforce but district health boards are still trying to determine the workforce requirements for the roll-out to groups three and four. The Ministry told us that it is providing additional support to district health boards to assist with workforce planning. This is positive. However, in our view there remains a risk that there will not be enough trained vaccinators available for the full scale-up.
5.11
Some information technology systems, including those that are required to support inventory management and distribution of the vaccine, are not scheduled to be fully implemented until shortly before the roll-out to groups three and four is due to start. Existing processes were expected to be sufficient for the current stage of the immunisation programme, but some of these – such as the inventory and distribution system – are already struggling with increasing volumes. Urgent work is required to get these new systems fully operational as soon as possible.
When will people receive their vaccine?
5.12
On 10 March 2021, the Government announced a detailed sequencing plan, which divided the population into four groups. See Appendix 2 for a summary of the sequencing framework and associated timing.
Equity was a central consideration in advice developed about sequencing
5.13
The Ministry set out to ensure that sequencing for the vaccine would be carried out in a fair, equitable, and consistent manner. Advice to Cabinet on 1 March 2021, which was based on input from the Independent Immunisation Advisory Group, recommended several changes to the initial sequencing framework to ensure equitable access to the Covid-19 vaccine. Higher morbidity, greater rates of hospitalisation, and earlier onset of degenerative diseases were identified as reasons to target Māori and Pasifika communities early. One of the changes recommended to Cabinet was that a risk-adjusted age (50 years) be used for Māori and Pasifika communities so that they could be included in group three. There was also a recommendation to adopt a whānau-centred approach to enable equitable outcomes.
5.14
That advice also highlighted that, in the past, immunisation campaigns have struggled to deliver for Māori and Pasifika, and that a “tailored approach, early engagement and active participation” were needed. To address this, it recommended that a proportion of the vaccine be allocated to Māori and Pasifika health care providers to distribute among Māori and Pasifika communities.
5.15
The advice also recommended that group two be expanded to include all staff and residents in long-term residential settings where there was a higher risk of transmission and severe outcomes from Covid-19. The rationale for this was that people in long-term residential settings have dual risks of a higher likelihood of underlying conditions and close contact living situations.
5.16
Cabinet agreed to provide a proportion of the vaccine (40,000 courses) to Māori and Pasifika health care providers to distribute to older people who might be living in hard-to-reach places and their households. Cabinet also agreed to expand the definition of those living in high-risk places.
People could be vaccinated later than they expect
5.17
As of 14 April 2021, district health boards plans showed that they anticipate having delivered about 1.1 million doses by the end of June 2021. This means that most of the people in group three will not be vaccinated before the end of June. Vaccination of groups three and four (due to start from July 2021) will overlap.
5.18
Although the Ministry told us that group four will start from July 2021, some planning documents show that most vaccines for group four will be administered between September and November 2021.
5.19
These timings are also subject to change if Pfizer does not deliver the vaccines as expected. The Ministry’s planning currently assumes a regular delivery of vaccines across the second half of the year.
5.20
In our view, the Ministry needs to be more transparent about when the public can expect to be vaccinated and the inherent uncertainties in the vaccine scheduling. This will help to manage expectations about how long people should expect to wait to be vaccinated.
More guidance is needed about how to apply the sequencing framework
5.21
The Ministry and district health boards are balancing the need to ensure that the vaccine goes to those who most need it first (in line with the sequencing framework) and ensuring that vaccine is not wasted. During the early stages of the roll-out, some sites found that up to 40% of their scheduled bookings did not arrive. This means it is necessary to have a clear strategy for how surplus vaccine doses will be used.
5.22
In the first few days of vaccinations in Auckland, there was public criticism of the way the district health boards offered surplus vaccine doses to staff members who were not front-line health workers. Other health workers, including those carrying out Covid-19 testing, felt they should have been offered the vaccine first, in accordance with the sequencing framework.
5.23
More recently, the media has reported that Canterbury District Health Board was offering surplus doses to employees of businesses located near its vaccination sites.
5.24
We were told that the Ministry does not want to turn anyone away from getting a vaccine, and district health boards are required to make provisions for walk-ins in their planning. The Ministry has issued guidance on managing surplus vaccine doses. District health boards are responsible for managing any surplus vaccine doses and the Ministry has recommended that to minimise wastage, district health boards should have a back-up or standby list of people to vaccinate that aligns to the sequencing framework. This guidance appears to relate primarily to group one and recommends that surplus vaccine doses ideally go to people in group two and preferably not group three.
5.25
The Ministry has stated publicly that it expects a degree of flexibility in how district health boards follow the sequencing framework. We understand that the Ministry has now told district health boards that the sequencing framework should be used as a guide but that they can distribute the vaccine in ways that best serve their communities and support equity.
5.26
In our view, this needs to be made clearer to members of the public so they understand what to expect ahead of the full roll-out to groups three and four.
| Recommendation 3 |
|---|
| We recommend that the Ministry of Health continue to improve guidance to district health boards about the scenarios in which it is acceptable to depart from the sequencing framework and make this transparent to the public. |
5.27
There might also be decisions to be made about sequencing for the general population in group four. Based on the current sequencing framework, all of group four will be eligible to be vaccinated from July 2021. This group includes about two million people. It is not clear whether further segmenting will occur (for example by age group). The Ministry told us that work on this is currently under way.
Where will people be vaccinated?
5.28 As at 18 March 2021, four types of locations for administering vaccines had been identified:
- hospital sites;
- temporary sites (such as workplaces, marae, churches, residential care facilities, and mobile clinics);
- community sites (such as GP hubs); and
- fixed-community vaccination centres.
5.29
A range of factors had to be considered for the design of the delivery settings. The settings needed to be varied enough to suit the needs of different target groups. At the same time, given the scale of the immunisation programme, logistical challenges, and tight time frames, there was value in having fewer setting types to simplify things.
5.30
Initial planning had to consider the storage requirements of a range of vaccine types. Although the decision to primarily use one type of vaccine simplified things, delivery models needed to be suitable for the Pfizer vaccine’s complicated requirements for storage and transportation at very cold temperatures. The range of settings that have been identified provide options for people to get vaccinated, but also make delivery more logistically complex.
5.31
Confirming the delivery and distribution models is a critical part of the roll-out planning. Other parts of the immunisation programme (such as the information technology and communications) also needed clarity about where and when vaccinations would happen to progress. Delivery models were not confirmed until mid-March 2021.
5.32
Delays in confirming the delivery models have been frequently identified as a risk to the immunisation programme. It has also affected some district health boards that felt this lack of preparedness meant they had to put some of their day-to-day work on hold to do urgent planning for the roll-out. Some of this might have been avoided if planning by the Ministry had been progressed earlier.
It is not clear that equity objectives will be fully achieved
5.33
The delivery models have been designed with equity in mind. Initially, the plan was to have fewer types of delivery locations. Advice from the equity team was that a wider range of location types would allow for more equitable access because they could be more tailored to each community’s needs – for example, by allowing mobile vaccinations or vaccinations in marae and churches. The early roll-out was also reviewed by a Māori health care provider who made recommendations on the types of sites that would be suitable. This advice was used to inform the design of the delivery models.
5.34
It was repeatedly highlighted in documents and interviews that a key lesson from the Covid-19 response and other immunisation campaigns is that Māori and Pasifika health care providers have the relationships with, and the trust of, their communities. Therefore, they should have a lead role in administering the vaccine.
5.35
A district health board-delivered model has benefits in that it can allow delivery to be locally tailored, which is potentially conducive to ensuring that there is equitable delivery. However, it also presents risks. We were told that district health boards have a variable track record with understanding and engaging Māori and Pasifika communities and health care providers. This means there could be inconsistency in delivery and approach for Māori and Pasifika communities.
5.36
To address this, the Ministry is working with district health boards to ensure that plans reflect how they have engaged with Māori and Pasifika providers, and how Māori and Pasifika providers can be involved in the vaccine roll-out. In the plans we saw from district health boards, the extent to which equity considerations were reflected was variable, with some providing detailed descriptions of what had and would be done, and others providing little detail.
5.37
Funding will also be provided directly to Māori and Pasifika health care providers ($11 million announced so far) to support them to build capacity to administer the vaccine (alongside additional funding for workforce development, which is discussed in paragraph 5.60). We were told this was done to address issues highlighted during the earlier Covid-19 response, where Māori and Pasifika health care providers were not given funding early enough to support preparations for Covid-19 response activities. As discussed in paragraph 5.16, there have also been 40,000 courses of the vaccine earmarked for Māori and Pasifika health care providers to distribute.
5.38
However, at the time we completed our audit, the Ministry had not yet finalised how the $11 million funding or the 40,000 courses of the vaccine for Māori and Pasifika health care providers would be distributed.
5.39
We heard that some Māori and Pasifika health care providers did not feel they were being properly engaged by district health boards. Delivering the vaccine involves a range of complex logistics and Māori and Pasifika health care providers will need time to prepare. The Ministry told us that 68 Māori and 25 Pasifika health care providers have now been engaged to support the roll-out. This is good progress, but in our view there remains a risk that, with a fast-moving programme, engagement with Māori, Pasifika, and disability health care providers does not receive sufficient priority as urgent roll-out tasks take precedence.
| Recommendation 4 |
|---|
| We recommend that the Ministry of Health continue to work with district health boards and Māori, Pasifika, and disability health care providers to make sure equity considerations are fully embedded in delivery plans. |
There remains a lack of clarity about the role of some providers and groups
5.40
The Ministry told us that, to some extent, all delivery models will be used in all stages of the roll-out. In the early stages there have been fewer vaccination sites, and vaccinations have focused on smaller target groups. As the immunisation programme progresses there will be increased priority on “volume over reach”, which the Ministry told us means vaccinating more people through existing sites. At the peak of the roll-out, it is expected that there will be many sites across many locations.
5.41
At the time of our audit, some district health boards we spoke to were still confused about the roles and responsibilities of other health care providers, particularly GPs, in the roll-out. One district health board had interpreted the Ministry’s guidelines to mean that only district health board staff could administer the vaccine, which meant GPs would not be involved.
5.42
Some groups, such as GPs, pharmacists, and nurses, told us they were unclear about their role. There was particular concern from some GPs that even if they were not involved in vaccinating, they would still be required to provide names of people needing to be vaccinated, which will place an administrative and financial burden on them.
5.43
As planning has progressed at the Ministry and in district health boards, there has been more clarity about how later stages of the roll-out will work. However, at the time we completed our audit this had still not been adequately communicated to GPs and other health care providers. We understand that the Ministry is working to strengthen engagement with GPs to explain how they will be involved at different stages of the roll-out. We strongly encourage the Ministry and district health boards to continue to engage with all relevant health care providers about their roles.
| Recommendation 5 |
|---|
| We recommend that the Ministry of Health provide more clarity to primary health care providers (including general practitioners) about their role in the wider roll-out to ensure that they have adequate time to prepare. |
How will vaccine supplies get to the vaccination sites?
5.44
The Pfizer vaccine is transported to New Zealand and stored in Auckland at -70˚C before it is delivered for vaccination (Figure 4). The vaccine is thawed and packed into smaller lots before distribution. Vaccine deliveries are dispatched in cold chain-certified Crēdo Cubes, accompanied by a security person. On arrival, the Crēdo Cubes are handed over to a named site receipt person. Medsafe has recently approved storing the vaccine at -20˚C for up to two weeks, which will allow more flexibility in how and where the vaccine can be stored.
Figure 4
How the Pfizer vaccine is delivered and stored
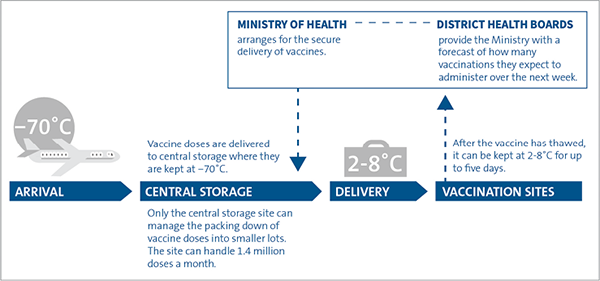
Source: Adapted from Ministry of Health guidelines.
5.45
This distribution approach has worked effectively so far. However, there is expected to be more pressure as the number of vaccinations increase. The capability and capacity of district health boards to forecast demand have been identified as a challenge. A paper to the Governance Group on 12 March 2021 highlighted the need for district health boards to have staff dedicated to managing site logistics, planning, and implementation. In April 2021, about 1000 doses expired and had to be destroyed by one district health board after more stock was ordered than was used. The Ministry told us this incident was reviewed and process improvements were implemented. It is continuing to work closely with logistics staff at district health boards to mitigate these risks.
5.46
Distribution logistics for the roll-out to groups three and four will become significantly more complex. As well as the increase in vaccination appointments, there will also be more vaccination sites to manage and vaccine doses will need to be transported across greater distances and to more remote locations.
5.47
Ensuring that supply does not exceed demand (which creates risk of wastage) needs to be balanced against ensuring that there is sufficient supply to cover larger than expected volumes (for example, when there are walk-ins). The Ministry is currently developing a more flexible “hub and spoke” distribution model that is expected to be in place by 1 June 2021.
5.48
In this hub and spoke model, a range of local storage hubs, where the vaccine can be stored at -70˚C or -20˚C, can be set up closer to vaccination sites. This should allow vaccines to be transported to vaccination sites faster and at shorter notice to respond to changes in demand. Work is also under way to determine which district health boards can manage local hubs and whether any private providers need to be contracted to do this.
5.49
In our view, these improvements are likely to be useful. However, there is still no system to record the level of vaccine stocks at each vaccination site in real time. Instead, this information is entered into spreadsheets after weekly stocktakes. The new logistics and distribution information technology platform is designed to resolve this. However, it will not be in place until 1 June 2021, after the roll-out to group three is scheduled to start. These systems are critical to ensuring that wastage of vaccine is minimised. Although wastage is currently relatively low, risks are likely to increase as the immunisation programme scales up.
5.50
District health boards and providers are responsible for ensuring that vaccine handling and storage requirements at vaccination sites are met. There could be some sites without facilities to store the vaccine appropriately (for example, a temporary site like a workplace, church, or marae). In these cases, the Ministry will arrange to transport the vaccine to the site daily.
5.51
The Ministry is also responsible for delivering the vaccine and consumables (such as syringes, needles, and saline) to vaccination sites. At the time of our audit, negotiations were still under way to procure further consumables to cover the remainder of the immunisation programme. District health boards and providers are responsible for sourcing other equipment not provided by the Ministry.
Who will administer the Pfizer vaccine?
5.52
The Pfizer vaccine will be administered by trained vaccinators. The Ministry is responsible for determining who can give the vaccine and managing how vaccinators will be trained. District health boards and providers are responsible for ensuring that they have enough trained vaccinators and other support staff.14
5.53
The current vaccinator workforce (a group that includes GPs, nurses, and pharmacists) is estimated to be about 12,000 people. Early estimates were that 1800 additional vaccinators would be required by June 2021, and 2000 to 3000 additional vaccinators would be required between August and November 2021. However, the estimates were based on an assumption that vaccinators would be working full-time, which is unlikely to be the case. In our view, more accurate and specific forecasting is still required.
5.54
Workforce capacity has been consistently identified as a risk throughout the immunisation programme, including by district health boards. It has been a challenge for district health boards to accurately forecast the workforce they need. The Ministry has developed a planning template for district health boards to estimate and report workforce needs. This template has been piloted and is intended to be in use from early May 2021. Although this is a positive development, it also means the Ministry and district health boards are still trying to determine the workforce requirements for the roll-out to groups three and four.
5.55
All vaccinators will need training to use the Covid-19 Immunisation Register, as well as training specific to Covid-19 and the Pfizer vaccine. This includes training on vaccine safety, the vaccination process, and how to respond to common concerns. The Ministry has contracted the Immunisation Advisory Centre to deliver this specific training. More than 4000 people have now been trained to administer the Pfizer vaccine, most of which are from the current vaccinator workforce.
5.56
Securing enough vaccination staff without diverting people from their roles in ways that will affect other health programmes (such as other immunisation programmes or public health work) is a challenge. The Ministry has been considering options for increasing the number of vaccinators by drawing from other groups and has approved additional health professions to participate in the vaccinator workforces. These different groups, which will all have different training needs, include:
- existing vaccinators (the practising regulated workforce);
- retired health workers and those who can currently vaccinate as part of their current roles, such as midwives, paramedics, registered nurses, pharmacists, and dentists (the non-practising regulated workforce); and
- those who are part of regulated workforces but who do not vaccinate as part of their current practice (such as physiotherapists and occupational therapists).
5.57
The Ministry has also decided to use the kaiāwhina workforce (which includes people working in non-regulated roles in the health and disability sector, such as health care assistants) as vaccinators.15 A tailored training pathway for this group is being finalised and is expected to start by the end of May 2021. In our view, this is good progress, but there is little lead-in time before the nationwide roll-out to recruit and train people from this workforce.
5.58
We heard that some in core health care workforces, such as nurses, felt they had not been properly engaged to be a core vaccinator despite their vast experience in vaccination and links with a wider range of communities. This was seen by them as an effect of the lack of early engagement with primary health care providers and Māori and Pasifika health care providers in the planning for the vaccine roll-out.
5.59
Part of supporting equity is ensuring that the vaccinator workforce is representative of the population it serves, and that vaccinations are administered by people trusted in the communities they work in. The lack of diversity in the vaccinator workforce has been identified as a problem, particularly its ability to reach and engage Māori and Pasifika communities.
5.60
Use of the kaiāwhina workforce in both vaccinator and support roles will likely support equitable uptake of the vaccine. At the time we completed our audit, specific funding for Māori and Pasifika workforce development had been promised, including $1.5 million for Māori workforce training and $24.5 million for a Māori vaccine support service. For Pasifika health care providers, the Ministry has set aside $750,000 for workforce training and $10.5 million for vaccination support. Although funding had been given to Pasifika health care providers, decisions were still to be made about how funding for Māori workforce development will be used. Funding to Māori health care providers is expected to be released by 1 July 2021, which is not long before group four is scheduled to start being vaccinated.
5.61
There has been good progress on training parts of the existing vaccinator workforce. We also acknowledge the efforts the Ministry is making to support district health boards in their workforce planning. However, at the time of our audit, the Ministry was still not clear on the overall workforce that will be required. In our view, identifying and securing sufficient and suitable workforce for the nationwide roll-out remains a key risk.
Information technology systems
5.62
Information technology systems are required to support identifying and inviting people to be vaccinated and getting the vaccine to the right locations. Four critical information systems are needed:
- an inventory and distribution system that organises and tracks the distribution of the vaccine and other consumables;
- a national booking system that supports people being invited and booked for a vaccine;
- a register that records all the clinical details of the vaccination; and
- a system that records any incidents of adverse events after vaccination.
5.63
To be fully effective, these systems need to be integrated with each other. For example, an immunisation register will record when someone needs their second vaccine dose, and the booking system will use that information to schedule the second appointment. For the inventory system to know how many doses of the vaccine to send, it needs to know how many people are scheduled to be vaccinated at a particular location. The adverse events system needs to be connected to the distribution system so it is ready for situations such as recalling batches of the vaccine.
A new Covid-19 Immunisation Register is now being used
5.64
The existing national immunisation register records patient data about immunisation programmes. The Ministry identified early on that this system would not be able to support the immunisation programme because it is not designed to handle the volume of people accessing it concurrently and it cannot collect all of the data required. Planning has been under way for an improved national immunisation register for several years but has not progressed.
5.65
The Ministry has now developed the Covid-19 Immunisation Register to hold a central record of who has been vaccinated. It can be accessed on the internet, which means it can be easily used in workplaces and community and mobile sites. The Covid-19 Immunisation Register is already being used to record vaccinations and it continues to be improved based on user feedback.
5.66
The Covid-19 Immunisation Register uses the National Health Index’s data to link to an individual’s health record. The National Health Index’s data includes a person’s National Health Index number, name, address, date of birth, gender, and ethnicity.
5.67
The immunisation programme also requires a system to invite people to be vaccinated and schedule their vaccinations. A national invitation and booking system that links with the Covid-19 Immunisation Register is being built. This system is expected to be completed by the end of May 2021, in time for the roll-out for group four.
5.68
In the meantime, district health boards are using their pre-existing booking systems. In some cases, these systems are rudimentary, for example, one is paper-based and others are simple Excel® spreadsheets. These systems are not designed for large-scale vaccinations. We were told that some district health boards have purchased new software for this purpose. There have been problems with some of these systems. In late March 2021, a privacy breach was discovered in the system Canterbury District Health Board used, which leaked the personal details of more than 700 people. We understand that the Ministry has carried out a review into the breach. It is unclear how district health boards’ systems might integrate with the national booking system, if at all. It is also anticipated that GP clinics will continue to use their own systems. In our view, the Ministry needs to work this through in more detail. If information is not brought together in one system, there could be co-ordination issues.
There will be challenges in identifying the right people to be vaccinated
5.69
It has been relatively straightforward so far to identify managed isolation and quarantine and other border workers to be vaccinated. This is because the Ministry of Business, Innovation and Employment holds a lot of this information through the Border Workers Testing Register.16 Even so, there have been some challenges. Some people may have become border workers since the roll-out started. For example, we were told that now that the travel bubble with Australia is operating, more staff at airports accepting international flights are classified as border workers. Since the first vaccine was administered, the number of people classified as border workers has increased from about 12,600 to 16,500. The Ministry of Health told us it relies on data supplied by employers to identify people in this group to be vaccinated.
5.70
As the vaccine is rolled out to more groups, there will be much less information about who those people are and how many are in each group. This will add to the challenge of monitoring progress with vaccine uptake and targeting areas where uptake is lower. We understand that the Ministry is developing a set of indicators to monitor progress towards equitable delivery.
5.71
The Ministry told us that it will monitor the uptake of different ethnic groups using the National Health Index. Ethnicity is recorded in the National Health Index and the Ministry told us it is sufficiently accurate for this purpose.
5.72
However, the National Health Index does not record information on whether a person has a disability or specific health condition. The Ministry is currently looking at whether a disability flag can be added to vaccination information using other existing datasets, and engaging with disability groups to try to estimate total numbers. This will need to be resolved if monitoring of uptake by specific groups, particularly people with disabilities, is to be effective. The Ministry and district health boards need to monitor uptake by priority groups to ensure that the vaccine is reaching them and address any gaps identified. It is important for people from priority groups to be represented in the uptake data so they have trust in the immunisation programme.
5.73
We were told that, when it is functional, the booking system will use email and text to invite people to be vaccinated. The Ministry is aware that this will make it difficult to reach some people and expects Māori and Pasifika health care providers and community groups to take the lead in inviting people in their communities to be vaccinated.
A distribution and inventory system will be deployed at the end of May 2021
5.74
At the time of our audit, the distribution and inventory system consisted of manual data entry into Excel® spreadsheets. The Ministry has recognised that this will not be sufficient for the roll-out to groups three and four. In our view, robust inventory management systems are essential to ensure appropriate supply to vaccination sites and to minimise wastage of vaccine.
5.75
A new distribution and inventory management platform is scheduled to be deployed at the end of May 2021. The proposed solution will support managing the distribution of vaccines and equipment from the -70˚C freezers located in one or more main hubs through to district health boards and other vaccination providers.
5.76
Planning for the number of doses of the Pfizer vaccine has factored in a wastage rate of up to 15% of the vaccine. This is a higher rate of wastage than normally expected on account of the difficult distribution and storage logistics for the Pfizer vaccine. The main methods district health boards have currently to manage the risk of wastage is planning for how surplus vaccine will be distributed and allowing walk-ins. It is expected that when a new distribution model is in place it will be easier to move the vaccine between sites at short notice, which will help with managing excess. Vaccine wastage can be calculated nationally, and at the end of April 2021 was estimated to be at 3.7%. It is intended that the new inventory management system will also allow wastage to be calculated at a local level.
The adverse reaction monitoring system is also being upgraded
5.77
Adverse reactions to medicines and vaccines in New Zealand are monitored by the Centre for Adverse Reactions Monitoring. The New Zealand Pharmacovigilance Centre (NZPhvC), which is a contracted service provider to the Ministry, manually receives and manages each report of an adverse drug reaction. The Ministry determined that this service would not be able to keep pace with the speed and scale of the vaccine roll-out.
5.78
The Ministry initiated a programme to bolster the service and made investments to create a separate Covid-19 adverse event management service within NZPhvC. The Ministry told us that this service is secure, scalable, and digitised, with full reporting and diagnostic capabilities and is integrated with the Covid-19 Immunisation Register.
There is a challenging time frame for finalising the information technology systems
5.79
Developing the final design of the information technology systems has relied on decisions made elsewhere in the immunisation programme, particularly decisions about sequencing and vaccination event types.
5.80
Although systems can be developed relatively quickly after these decisions are made, it puts a strain on the time frames. Some aspects of information technology systems are being finalised “just in time” for each stage of the roll-out. For example, the national booking system and new distribution systems are both scheduled to be deployed shortly before the roll-out to group four, which leaves little time for addressing any issues that arise. At the time of our audit there was still a lot of work to do to integrate these systems.
5.81
The Ministry has identified a range of risks relating to the security and privacy of information. It told us controls were in place to manage these risks now, and that further controls would be added. Our work has not included testing of the controls the Ministry has in place.
5.82
We understand that the controls in place relate to the national information technology systems. If district health boards and the other organisations involved use their own systems to support vaccinations, they will be responsible for having the necessary privacy and security protections in place for their own systems. This includes any booking systems used by district health boards in advance of a national booking system being available.
5.83
It is not clear how the Ministry is assessing the robustness of systems to address vaccination volumes and load, although we note that cloud-based systems are scalable. The volume the systems will need to be able to manage and how the information technology will work will also be influenced by sequencing decisions. Although decisions have been made about the overarching sequencing framework, decisions still need to be made about whether and how vaccinations for the rest of the population will be sequenced. The approach that is taken (for example, based on different age groups or locations) could influence aspects of the booking system design.
14: The vaccinator is only one part of the workforce needed. Successfully vaccinating people also requires an available range of support staff, including receptionists and the kaiāwhina workforce, which includes people working in non-regulated roles in the health and disability sector, such as health care assistants. These staff are needed to support people through the vaccination process.
15: A 12 March 2021 update to the Governance Group noted that the current legislation does not restrict kaiāwhina health professionals from administering vaccines.
16: This is the register that is used to record regular Covid-19 testing for border workers.